DO U KNOW TO CREATE ELECTRICITY FROM A LEMON???
By Rajlish
@Rajlish (7)
India
November 18, 2006 4:57am CST
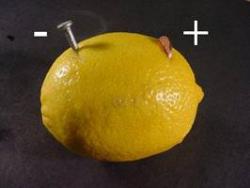
Creating a battery from a lemon is a common project in many science text books. Successfully creating one of these devices is not easy. Batteries consist of two different metals suspended in an acidic solution. Copper and Zinc work well as the metals and the citric acid content of a lemon will provide the acidic solution. Batteries like this will not be able to run a motor or energize most light bulbs. It is possible to produce a dim glow from an LED.
The lemon: A large, fresh, "juicy" lemon works best.
The nail: Galvanized nails are coated in zinc. I used a 2" galvanized common nail.
The penny: Any copper coin will work. (Canadian pennies from 1960 - 2001 all worked)
2 responses